https://www.pv-magazine.com/2022/10/28/latest-advances-in-sodium-ion-battery-research/
Latest advances in sodium-ion battery research
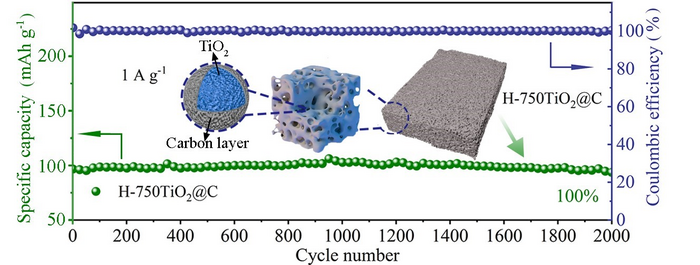
Image: Qingdao University
Anatase titanium dioxide (TiO2) is a promising anode material for sodium-ion batteries due to its low cost, non-toxicity and abundance. However, the low electronic conductivity and sluggish ion diffusion kinetics at high rates are holding back practical applications.
A team of researchers from the College of Materials Science and Engineering at Qingdao University in China has demonstrated a sol-gel approach to the synthesis of thermally stable anatase nanoparticles with a carbon shell as anode materials for sodium-ion batteries.
When the synthesized anatase was tested in a half battery, the researchers found that the battery exhibited a reversible specific capacity of 228 mAh g-1 – at a current density of 0.05 A g g-1 with 100% capacity retention after 2,000 cycles at 1 A g-1.
In-situ X-ray diffraction and Raman spectroscopy results have revealed a nearly zero-strain characteristic of anatase during charge/discharge processes. In-situ transmission electron microscopy, ex-situ X-ray photoelectron spectroscopy and scanning electron microscopy results suggested an irreversible sodiation-activation process to form a sodiated-TiO2 phase during the initial discharge process.
A full coin cell assembled with anatase as the anode and Na3V2(PO4)3 as the cathode delivered an energy density of 220 Wh kg.The scientists reported their results in “Sodium-ion Storage Properties of Thermally Stable Anatase,” which was recently published in Energy Material Advances.
Operando analysis
Popular content
A joint research group from Helmholtz-Zentrum Berlin (HZB) and Humboldt-Universität zu Berlin, meanwhile, has used operando techniques to observe how solvated natrium ions embed themselves in electrodes.
The storage of ions, when accompanied by their solvation shell in a crystal lattice, is referred to as “co-intercalation.” Up to this point, this concept was limited to the negative electrode of the sodium-ion battery. Now, Berlin-based researchers have succeeded in extending the concept to the positive electrode of the battery.
“With titanium disulphide (TiS2) and graphite, we have for the first time combined two materials that absorb and release the same solvent during charging and discharging of the battery,” said Guillermo A. Ferrero, one of the researchers behind “Co-intercalation batteries (CoIBs): Role of TiS2 as electrode for storing solvated Na ions,” which was recently published in Advanced Energy Materials.
The scientists could observe changes in the material during charging and discharging via operando measurements performed in the X-Ray Core Lab at HZB on the LIMAX 160. This helped them to assign the co-intercalation mechanism inside the battery.
“The process of co-intercalation could improve upon efficiency by enabling better low temperature performance,” said Katherine A. Mazzio of HZB. “It could also be utilized to improve upon alternative cell concepts such as using multi-valent ions instead of Li+ or Na+ storage that are particularly sensitive to the solvation shell.”
This content is protected by copyright and may not be reused. If you want to cooperate with us and would like to reuse some of our content, please contact: editors@pv-magazine.com.